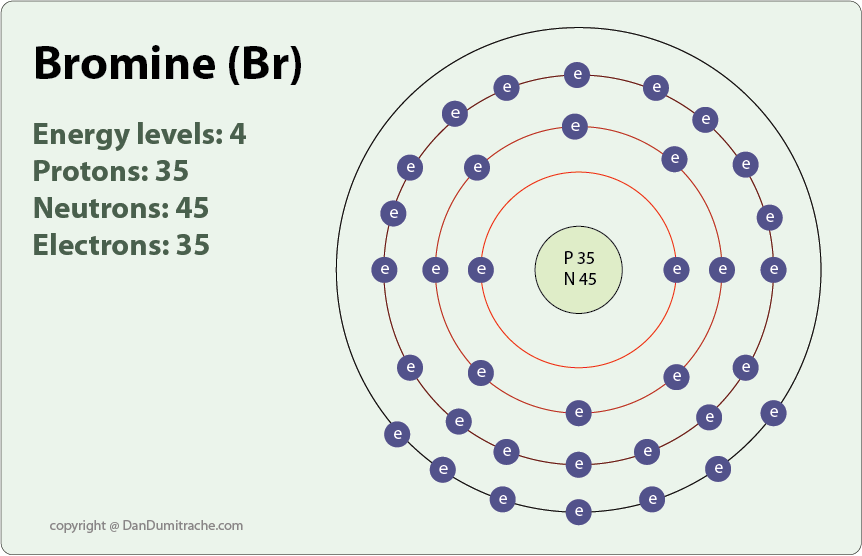
Bromine Z35 with 35 electrons can be found in Period 4 Group VII of the periodic tables. What is the Lewis dot diagram of hydrogen bromide.

How Many Valence Electrons Does Bromine Br Have Valency Of Bromine
You need 2 electrons for a bond so you end up with H bonded to Br and 6 electrons around Br.

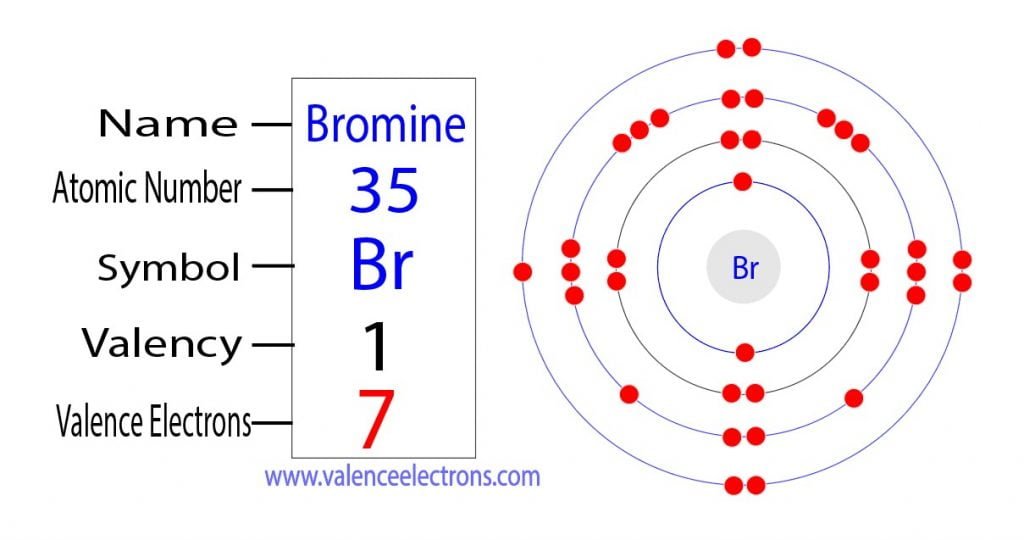
. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Lets look at the bromine atom to understand this principle. Up to 24 cash back In the case of Bromine the abbreviated electron configuration is Ar 3d10 4s2 4p5.
The total number of electrons present in the valence shell of an atom are called valence electrons and there are a total of seven electrons. What is the bond between Br atoms in a Br2 molecule. Br will have eight valence electrons and a negative charge.
Covalent formed by the sharing of two valence electrons. You need 2 electrons for a bond so you end up with H bonded to Br and 6 electrons around Br. Another way to find the valence electrons is by the group number the atom is found inBr is in group 7A of the periodic table which means it has 7 valence electrons as all other atoms in.
Counting the 4th shell orbitals and their electrons Bromine has two 4s electrons and five 4p electrons giving it a total of 7 valence electrons. The electronic configuration of Bromine is 1s22s22p63s23p64s23d104p5 and the valence electrons are in the 4s and 4p orbitals giving Bromine 7 valence electrons. Hence both bromine and fluorine have seven valence electrons.
Since in each halogen atom we have 7 valence electrons Hence they will try to get 1 more electron. The first is to use the Periodic Table to figure out how many electrons Bromine h. During the formation of a bond the last shell of bromine receives an electron and turns into a bromide ionBr.
Ok but how many valence electrons does an atom of Bromine have. Br has 7 valence electrons and H has 1 valence electron so thats 8 in total. In the periodic table both bromine and fluorine lie in group 17.
Up to 24 cash back How many valence electrons in Bromine. Since BrF 5 has one bromine atom and. Br has 7 valence electrons and H has 1 valence electron so thats 8 in total.
There are two ways to find the number of valence electrons in Bromine Br. If you want to find the valence electrons of bromine from its electron configuration then you should know its electron configuration. 119 rows Valence electrons.
The elements that receive electrons and form bonds are called anions. Up to 24 cash back Bromines electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 shows that the third energy level is full with 18 electrons 3s2 3p6 3d10 and therefore. Counting the 4th shell orbitals and their electrons Bromine has two 4s electrons and five 4p electrons giving it a total of 7.
For each bond show the direction of polarity by selecting the correct. Br has 7 valence electrons and H has 1 valence electron so. This means that the central Bromine Br atom will have an odd number 9 total.
Bromine is 7 valence. For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit. From the Electron Configuration.

How Many Valence Electrons Does Bromine Have Youtube
Chem4kids Com Bromine Orbital And Bonding Info
Why Does Fluorine Have A Higher Ionization Energy Than Bromine Socratic
0 Comments